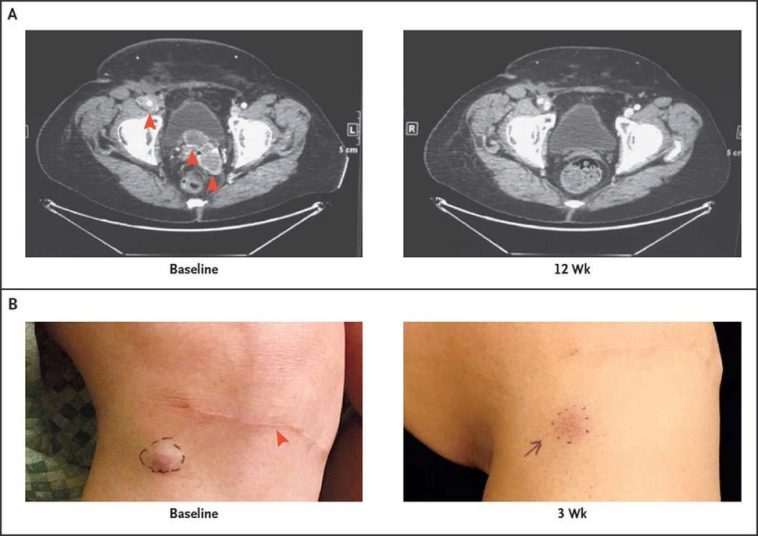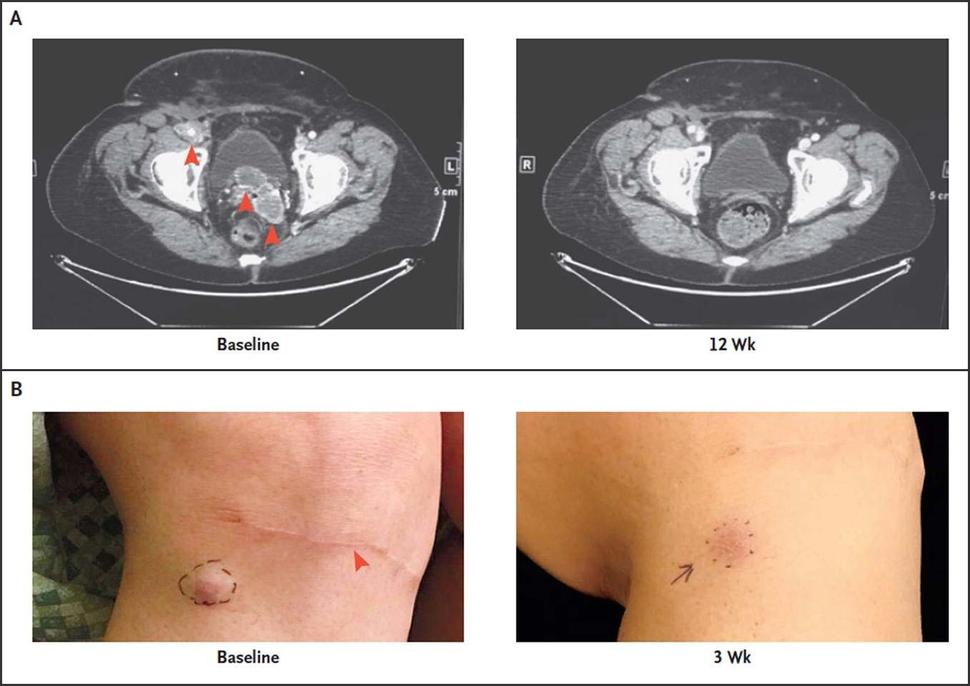

Investigating Merkel Cell Cancer Clinical Trials: An Opinion Editorial
Clinical trials have long been a cornerstone in the quest to improve treatment and outcomes for various cancer types. Merkel cell cancer, a rare but aggressive form of skin cancer, is no exception. In this opinion piece, we will take a closer look at Merkel cell cancer treatment through the lens of clinical trials, sharing our perspective on emerging opportunities, tricky parts, and important considerations for patients and caregivers alike.
As someone who has followed medical progress in both modern and alternative arenas, I firmly believe in the importance of clinical research. In this article, we will poke around at the various phases of these studies, explore how trials are structured, and examine why it is essential to be well-informed when considering participation in a trial. We will also use tables, bullet lists, and subheadings featuring popular, detailed phrases that matter to anyone facing this diagnosis.
Understanding Merkel Cell Cancer: The Basics and Beyond
Merkel cell cancer is a rare but dangerous skin cancer known for its tendency to spread rapidly. Although its occurrence is less frequent when compared to other skin cancers, the aggressive nature of Merkel cell cancer makes early detection and innovative treatment strategies especially critical.
One of the key advantages of clinical trials is that they are designed to test new methods for early detection, prevention, and treatment, offering hope where conventional therapies can fall short. In this opinion editorial, I am here to express that clinical research truly shines a light on the most promising avenues for treatment, despite the many confusing bits along the way.
Clinical Trials: A Closer Look at How They Work
Before diving in into the specifics of Merkel cell cancer trials, it is important to outline the structure of clinical trials in general. Clinical trials are research studies involving patients and are supported by various organizations and governmental institutions, including the National Cancer Institute (NCI). These studies not only test the effectiveness of new treatment approaches but also work to improve our understanding of how different therapies interact with the human body.
There are several phases in a clinical trial, each designed to answer specific questions about the experimental treatment:
- Phase I: This phase focuses on finding safe dosage levels and identifying any immediate side effects. The group of participants here is usually small, meaning that the data generated may be limited but is absolutely necessary to ensure patient safety.
- Phase II: After establishing safety, researchers then measure how effective the treatment is in a larger group of people. It’s in this phase that the tricky parts of dosage optimization and efficacy often become apparent.
- Phase III: This phase involves testing the new treatment on large groups of patients to compare it against the current standard treatments. The outcomes here are pivotal in determining if a new treatment should become a standard practice.
- Phase IV: Post-marketing studies are conducted to track the long-term effects of the treatment in a broader patient population. These studies are key to understanding the fine points and little details that might otherwise be missed in earlier phases.
Each stage raises its own set of complicated pieces, and understanding these can significantly help patients make a better-informed decision when considering enrollment in a trial.
Clinical Trials for Merkel Cell Cancer: Hidden Details and Benefits
Clinical trials targeted at Merkel cell cancer treatment are both exciting and nerve-racking for several reasons. On the plus side, they offer patients access to cutting-edge therapies and the possibility of contributing to advancements that may benefit future generations. However, the journey through a clinical trial is also full of problems, twists and turns, and subtle details which might seem overwhelming at first glance.
The benefits of participating in these clinical trials include:
- Access to new therapies that may not be readily available otherwise.
- Contributing to medical research that can lead to breakthroughs in treatment not only for Merkel cell cancer but also for other malignancies.
- Close monitoring by experts in the field, which can be especially key given the aggressive nature of Merkel cell cancer.
- The opportunity to potentially avoid more harmful side effects associated with standard treatments.
On the flip side, patients must also consider the possibility of receiving a placebo or experiencing unexpected side effects from experimental treatments. Therefore, it is super important for patients to speak openly with their doctors when weighing these options.
Key Questions to Consider Before Joining a Clinical Trial
The decision to join a clinical trial is not one that should be taken lightly. If you or a loved one is considering this option, here are some essential questions to ponder:
- What is the trial’s purpose, and what are its specific aims in treating Merkel cell cancer?
- What are the inclusion and exclusion criteria for participation? Understanding these limitations can help determine if you or your loved one qualifies.
- How will the trial impact your day-to-day life, including routine check-ups and potential lifestyle changes?
- What are the possible side effects, and how do these compare with current standard treatments?
- Are you comfortable with the level of oversight provided by a trial, and do you trust the expertise of the clinical team?
These questions are intended to help figure a path through some of the tangled issues that surround clinical trials, ensuring that everyone involved feels informed and empowered to make the best decision for their health.
Expert Perspectives on Clinical Research for Merkel Cell Cancer
Medical experts often have varied opinions when it comes to recommending participation in clinical trials. Many suggest that clinical research is a double-edged sword: while it opens up exciting opportunities, it also brings along a fair share of complications and confusing bits. Here are some of the expert viewpoints on this subject:
- Innovative Treatment Approaches: Experts argue that Merkel cell cancer trials provide an opportunity to test novel therapies that might prove more effective than existing treatment regimens. These innovations are sometimes born out of alternative or complementary medicine practices, making it an intriguing space for cross-disciplinary collaboration.
- Risk Versus Reward: On the risk side, experts acknowledge that new treatments could come with unexpected side effects. The key, however, lies in ensuring that patients are fully informed and that there is continuous monitoring by medical professionals throughout the study’s duration.
- Enhancing Medical Understanding: Beyond individual treatment, clinical trials help researchers gain intimation into how Merkel cell cancer behaves. This is crucial not only for current patients but also for developing future strategies that improve overall cancer care.
When you take a closer look at the minimal details and subtle distinctions within these expert analyses, it becomes clear that involvement in a clinical trial is a personal choice, albeit one that could lead to groundbreaking improvements in care.
Making Your Way Through Clinical Trial Options
For many patients grappling with a severe diagnosis, the idea of joining a clinical trial might seem off-putting or even intimidating. However, with adequate guidance and a firm grasp on the subject matter, managing your way through the available options becomes a more approachable task. Here are some balanced perspectives and strategies to help you get around the tricky parts of this decision:
- Detailed Research: It is essential to gather as much information as possible. This includes reading reliable sources, talking to healthcare professionals, and, if possible, connecting with past participants of the trial.
- Risk Awareness: Recognize that every treatment option comes with its own set of risks. Dive in into the data available regarding side effects, success rates, and reasons for trial discontinuation.
- Support System: Do not underestimate the value of emotional and social support from family, friends, or support groups. Their insights are often based on real-world experiences that can complement your medical research.
- Doctor’s Guidance: Speak to your primary care provider and oncologist. Their advice, tailored to your unique health profile, is paramount in making such a decision.
Figuring a path through this labyrinth requires balancing hope with realism. The subtle parts of trial participation—from the fine details in the consent forms to the hidden complexities of follow-up visits—are best tackled with informed support.
Comparing Treatment Options: Standard Care Versus Clinical Trials
The landscape of cancer treatment continuously evolves, and one of the most nerve-racking choices for patients is whether to pursue the standard care route or take the leap into a clinical trial. Here’s a brief comparison in a tabular format to help clarify some of the key distinctions:
| Aspect | Standard Care | Clinical Trial |
|---|---|---|
| Treatment Access | Widely available as part of conventional treatment regimens. | Access to experimental treatments and potentially groundbreaking therapies. |
| Monitoring | Regular check-ups and standard monitoring procedures. | Intensive monitoring by experts to track side effects and effectiveness. |
| Risk Factors | Well-documented side effects with extensive usage history. | Potential for unknown side effects given the experimental nature. |
| Contribution to Research | No direct impact on future research developments. | Helps shape future treatment protocols and enhance scientific knowledge. |
| Flexibility | Treatment plans are largely fixed and standardized. | Protocols may be adapted as new data emerges during the study. |
This comparison highlights that while standard care has its benefits rooted in a long history of research and application, clinical trials offer an opportunity to be part of something innovative—even if it means facing some overwhelming and nerve-racking challenges along the way.
Balancing Hope and Reality in the Clinical Research Journey
It is undeniable that the prospect of living with Merkel cell cancer is intimidating. However, the clinical trial pathway can also represent a door to hope—a chance to benefit from the latest research findings and treatment innovations. Like any medical journey, the decision to participate requires a careful measurement of risk versus reward.
The hope provided by clinical research is grounded in rigorous experimentation and the collective efforts of the medical community to solve the tangled issues associated with cancer treatment. Even if the process is loaded with issues and moments that seem nerve-racking, every step taken contributes to a larger picture where significant breakthroughs become possible.
For many patients facing a serious diagnosis, the clinical trial experience is also an invaluable learning opportunity. It is a chance to work through challenges, navigate confusing bits in treatment protocols, and ultimately participate in creating a better standard of care for future patients. The ongoing research in this field is a testament to the resilience and innovative spirit of both the medical community and the patients who take part.
Patient Stories and Personal Reflections
One of the most powerful aspects of clinical trials is the individual story behind each participant. Although medical data and research statistics are critical, the personal narratives that emerge from clinical trials bring an essential human element to the discussion.
Consider the story of a patient who, after exhausting standard treatment options, chose to enroll in a Merkel cell cancer clinical trial. This individual detailed the small distinctions between daily life before and after joining the trial. Initially, the decision was daunting—filled with nerve-racking uncertainty—but over time, the patient noticed the benefits of intensive monitoring and the opportunity to be actively involved in their treatment plan. These personal reflections underscore that, despite the twists and turns inherent in clinical research, the potential benefits can indeed be life-changing.
From a personal perspective, I find that these real-life accounts help demystify the complex pieces of clinical research. They encourage others to get into a conversation with their doctors and to consider the possibility that participation in a clinical trial might be the necessary next step on the road to recovery or improved quality of life.
Alternative Perspectives: Complementing Modern Medicine with Integrative Approaches
In many cases, patients benefit from not only modern therapeutic options but also integrative approaches that include elements of alternative medicine and nutritional support. Clinical trials naturally align with this holistic view, especially when further research into Merkel cell cancer unveils opportunities to combine treatments. This may involve blending cutting-edge immunotherapies or targeted treatments with complementary practices known to boost overall well-being.
For example, nutritional interventions that optimize the immune system or reduce inflammation might be explored alongside experimental drugs in clinical trial settings. Here are some ways integrative approaches can be integrated with clinical research:
- Dietary Adjustments: Incorporating diets rich in antioxidants or anti-inflammatory foods can sometimes alleviate symptoms and boost the effectiveness of cancer treatments.
- Physical Activity: Regular exercise, tailored to the patient’s capability, can improve overall health and reduce stress, making the body more resilient during therapies.
- Mind-Body Techniques: Practices like meditation, yoga, or even acupuncture might help alleviate side effects, reduce anxiety, and improve the patient’s quality of life.
- Herbal and Natural Supplements: While these should never replace conventional treatments, they can sometimes provide additional support when used under strict medical supervision.
These complementary approaches do not replace the need for rigorous clinical studies but rather enhance a patient’s capacity to endure and respond to treatment. Patient feedback and personal stories continue to highlight just how significant combining traditional and alternative methods can be in managing the disease.
Practical Steps and Tips for Prospective Trial Participants
For patients considering joining a Merkel cell cancer clinical trial, taking a structured, step-by-step approach can help demystify the process. Here are some practical steps and tips that can guide you through the various stages of participation:
- Research Thoroughly: Begin by reading up on the specifics of the trial. Understand its purpose, eligibility criteria, treatment protocols, and the anticipated outcomes.
- Consult with Specialists: Don’t hesitate to get a second opinion. Talking with multiple healthcare professionals can provide diverse perspectives on whether the trial is the best option.
- Weigh the Risks and Benefits: Make a detailed list of the potential advantages and possible side effects, then prioritize based on your health priorities and personal circumstances.
- Engage with Support Groups: Look for forums or local support groups where former participants share their experiences. These firsthand accounts can offer valuable insights into what to expect.
- Prepare Mentally and Emotionally: Recognize that joining a clinical trial is a significant decision—and sometimes a nerve-racking one. Preparing yourself with a robust support network can make the journey smoother.
Below is a summarized checklist for prospective participants:
| Checklist Item | Action |
|---|---|
| Information Gathering | Read the trial protocol, research study background, and latest published data. |
| Medical Consultation | Set up meetings with your primary doctor and oncology specialist to discuss potential eligibility and expectations. |
| Risk Assessment | List potential side effects and weigh them against the benefits of accessing new treatments. |
| Emotional Support | Engage with support groups, counselors, or trusted family members. |
| Informed Consent | Review all provided documents closely, ask questions, and ensure you fully understand the trial details before signing. |
These actionable steps are designed to help patients steer through both the subtle details and high-stakes decisions that clinical trials often require. Approaching everything with open communication and a willingness to ask for help is key.
Future Directions in Merkel Cell Cancer Research
As more clinical trials are launched, there is a growing sense of optimism among researchers and practitioners about the potential to outsmart Merkel cell cancer. The future of this research appears promising, with studies currently exploring novel treatments that combine immunotherapy, targeted drugs, and even regenerative medicine techniques. These cutting-edge concepts are gradually being tested and refined in real-world clinical settings.
What makes this an especially attractive field of study is the dedication among researchers to not simply accept the status quo but to continuously challenge it. This means that while patients might have to deal with several nerve-racking moments along the way, every trial contributes important insights that help shape tomorrow’s standard of care.
In addition, collaboration across disciplines—be it modern medicine, alternative therapy, or nutritional science—promises to further enhance treatment outcomes. The collaborative approach ensures that the small distinctions in treatment protocols, which might seem like mere side notes, are being thoroughly investigated to maximize patient benefit.
Final Thoughts: Embracing a Comprehensive Perspective in Cancer Treatment
In wrapping up this reflective exploration into Merkel cell cancer clinical trials, I encourage patients, caregivers, and medical professionals to view clinical research as both a challenge and an opportunity. While the path is dotted with complicated pieces and nerve-racking decisions, it ultimately paves the way for revolutionary advances in treatment.
By taking a balanced approach—one that looks at both the innovative potential and the inherent risks—patients can make better-informed decisions. It is essential to remember that every trial not only tests new treatments but also enriches our overall understanding of this aggressive disease. Each study is a stepping stone toward improving survival rates, easing treatment side effects, and, ultimately, instilling hope for a healthier future.
The road to conquering Merkel cell cancer is full of hidden complexities and fine points that require a multidisciplinary effort. Whether through rigorous clinical research or the integration of holistic practices, the journey demands patience, resilience, and careful navigation. Everyone involved—from the patient to the clinician—plays a critical role in this shared endeavor.
In my view, participation in a clinical trial emerges not solely as a personal treatment decision but also as a societal contribution: an off-beat step toward reshaping the future of cancer therapy. While the journey may at times seem overwhelming, remember that every challenge faced is a contribution to a larger mission—a mission aimed at turning experimental hope into established, life-saving treatments. Let us embrace the twists and turns of clinical research, stay informed, and continually push for advancements that make a real difference in the lives of those battling Merkel cell cancer.
Ultimately, the future of cancer care lies in our ability to combine empirical research with human empathy, and clinical trials are the proving grounds where this potent blend is put into practice. Together, the medical community and patients can transform today’s challenges into tomorrow’s breakthroughs, making the trial process not just a test of treatment, but a testament to our commitment to conquering cancer for good.
Originally Post From https://www.cancer.gov/research/participate/clinical-trials/disease/merkel-cell/treatment?pn=1
Read more about this topic at
Clinical trials for Merkel Cell Carcinoma
Treatment Clinical Trials for Merkel Cell Cancer