Optimizing Treatment for Metastatic Castration-Resistant Prostate Cancer: A Closer Look
The management of metastatic castration-resistant prostate cancer (mCRPC) stands as one of modern medicine’s most challenging arenas. Despite significant advances in therapy, many clinicians and patients find themselves trying to figure a path through a maze of intricate treatment options. At its core, mCRPC is driven largely by androgen receptor (AR) signaling, and while current Food and Drug Administration-approved therapies have extended survival, many troublesome questions remain about the best ways to beat resistance. This opinion piece takes a closer look at the tough, tangled issues of treatment optimization, examines emerging strategies, and emphasizes the importance of using biomarkers to tailor therapy in this evolving field of precision oncology.
Before diving in, it’s important to acknowledge that in the realm of mCRPC, both clinicians and patients face a landscape loaded with issues, such as the tricky parts of resistance mechanisms and the overwhelming task of selecting and sequencing available therapies. In this editorial, we will explore the key approved therapies, emerging treatment options that promise to shift current paradigms, and detailed approaches using biomarker-driven insights to further personalize care.
Current FDA-Approved Therapies: The Established Arsenal
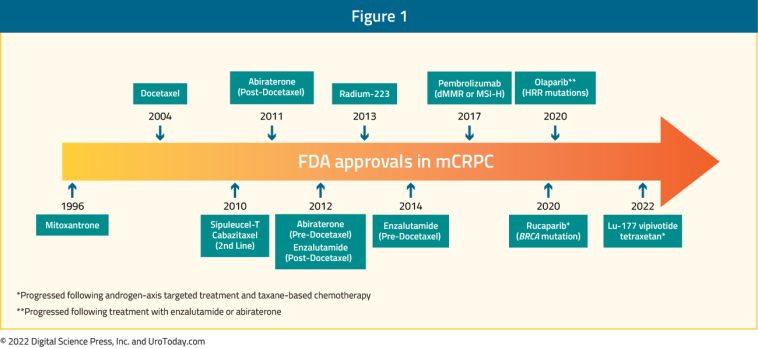
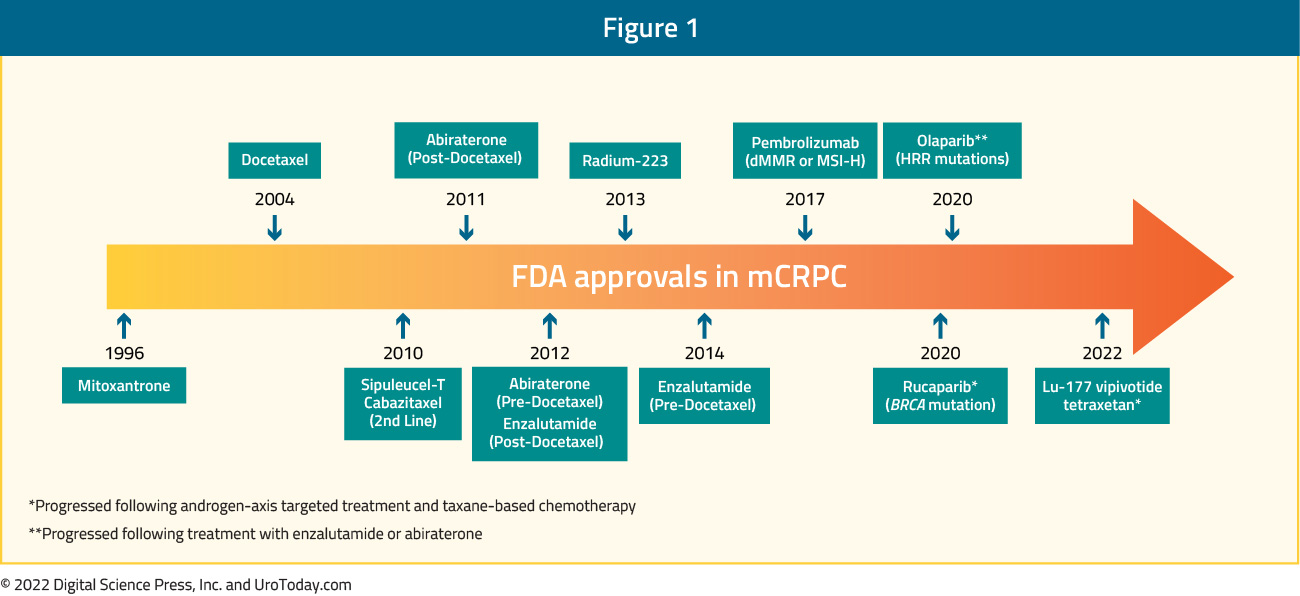
Over the past decade, regulatory agencies including the US Food and Drug Administration have approved a variety of agents for the management of mCRPC. These therapies include next-generation androgen receptor signaling inhibitors (ARSIs), poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors, radioligand therapies, and even immunotherapy. Each of these approved strategies represents a key component of the modern therapeutic approach and has contributed to an improvement in overall survival for patients with mCRPC.
Next-Generation AR-Signaling Inhibitors
AR-signaling inhibitors have long been a cornerstone in addressing the AR-driven progression of prostate cancer. These medications are engineered to disrupt the AR pathway, a strategy that directly interferes with the signals that promote cancer cell survival. Yet, even with their success, many patients eventually experience a resurgence in disease activity, reflecting a range of resistance mechanisms that clinicians must contend with.
Some of the tricky parts in dealing with AR-signaling inhibitors include:
- Understanding the subtle details of AR mutations and splice variants
- Managing cross-resistance between different AR-directed agents
- Identifying patients who might benefit best from switching strategies
These complicated pieces reflect the many twists and turns inherent in AR pathway targeting. Yet, physicians continue to refine treatment regimens as they gain insights from ongoing clinical trials and emerging research.
PARP Inhibitors
Poly(ADP-ribose) polymerase inhibitors have emerged as a promising class of agents in the treatment of mCRPC. Their approval was based on the discovery that a subset of mCRPC patients harbors defects in homologous recombination repair genes, making them particularly vulnerable to DNA repair inhibition. Despite their effectiveness, the incorporation of PARP inhibitors into clinical practice demands a deep understanding of the genetic background of the tumor.
To make the best use of these drugs, clinicians need to consider factors such as:
- The patient’s genomic profile, especially in relation to DNA repair gene mutations
- The timing and sequencing of PARP inhibitors with AR-directed therapies
- Potential side effects and the need for careful monitoring
The challenges here are not insurmountable, but they do require a steady commitment to genetic profiling and personalized care. The integration of PARP inhibitors into treatment paradigms illustrates how precision oncology can figure a path even in complicated clinical scenarios.
Radioligand Therapy and Immunotherapy
Another important chapter in the story of mCRPC treatment comes from radioligand therapy. Using molecules that combine a radioactive payload with a ligand that specifically homes into cancer cells, this approach directly delivers radiation to tumor sites. Although still emerging as an essential piece of the therapeutic puzzle, radioligand therapy offers hope for patients with advanced stages of the disease.
Similarly, immunotherapy is being investigated as a strategy to harness the body’s own immune system against mCRPC. Even though immune-based therapies have changed the landscape for several other types of cancer, their role in mCRPC is still being shaped. The trial-and-error nature of these newer therapies means that clinicians must work through a series of challenging bits when integrating them into practice.
Current Treatment Challenges: Sequencing and Resistance
While FDA-approved therapies have provided an expanded therapeutic arsenal, their optimal sequencing remains one of the more nerve-racking issues in the field. Choosing when to use AR-signaling inhibitors, PARP inhibitors, radioligand therapy, or immunotherapy involves balancing survival benefits against potential side effects and costs. Resistance mechanisms—whether through AR gene mutations, activation of alternative signaling pathways, or cellular survival tactics—lead to a need for constant reevaluation of treatment strategies.
Key factors complicating treatment sequencing include:
- Determining the right time to change therapy after initial responses wane
- Understanding how one treatment might affect the efficacy of another
- Dealing with mixed responses in patients due to the heterogeneity of cancer cells
These little details in treatment interaction require clinicians to take a closer look at each patient’s progression and adjust their regimens accordingly. The practice of setting a universal guideline is tough because of the many small distinctions that come into play with each new patient scenario.
Emerging Therapeutic Strategies: New Frontiers in mCRPC Management
Even as we rely on the effectiveness of approved drugs, the research community is busy exploring novel approaches that might offer greater benefits. These innovative strategies are particularly promising for patients whose cancers do not adequately respond to current treatments. Let’s explore some of the front-runner emerging therapies that are currently making waves in clinical trials and early clinical use.
Androgen Receptor Degraders
One of the most exciting strategies in the works is the use of AR degraders. Unlike traditional AR inhibitors that block the receptor’s function, AR degraders work by actively breaking down the androgen receptor protein. This approach may help overcome some of the compensatory mechanisms that cancer cells use to sidestep inhibition.
Some of the key points regarding AR degraders include:
- Mechanism: They target the receptor for destruction rather than simple blockade.
- Potential Benefits: May prevent the emergence of resistant clones that thrive under conventional AR inhibition.
- Translational Potential: Early data are promising, although larger trials are needed for conclusive evidence.
The appeal of AR degraders lies in their potential to add an extra layer of control over AR signaling, a key driver of mCRPC. Many experts believe that incorporating these agents into treatment regimens could mitigate some of the tangled issues associated with acquired resistance.
Antibody-Drug Conjugates (ADCs)
Another innovative approach being considered is the use of antibody-drug conjugates or ADCs. This strategy involves coupling an antibody specific to a protein expressed on cancer cells with a potent chemotherapeutic agent.
ADCs have several advantages in the context of mCRPC:
- Targeted Delivery: By directly targeting cancer cells, ADCs minimize damage to surrounding healthy tissue.
- Enhanced Efficacy: The direct delivery of the cytotoxic agent helps overcome some of the limitations of traditional chemotherapy.
- Flexibility: ADCs offer the possibility to tailor treatment based on the expression of specific cell surface markers.
These strategies are promising, though they require careful patient selection and a precise understanding of the tumor’s surface protein profile. The use of ADCs underscores the shift toward more tailored treatment options where therapy is closely matched to the biology of an individual’s cancer.
T-Cell Engagers and Immunotherapy Advances
While traditional immunotherapy approaches have yet to fully translate into the mCRPC arena, recent developments in T-cell engagers are showing potential. T-cell engagers are designed to bridge immune cells with cancer cells, effectively rallying the body’s own defenses to recognize and destroy the tumor.
The use of T-cell engagers in mCRPC involves:
- Mechanism Integration: Combining these agents with existing immune checkpoint inhibitors may boost the overall immune response.
- Patient Selection: Identifying the appropriate candidates by exploring tumor antigen expression and other biomarkers.
- Overcoming Resistance: These agents can potentially shift the immune landscape in tumors that are loaded with immune evasion tactics.
Even though the path toward wide clinical acceptance is still full of problems, these novel immunotherapy strategies offer a beacon of hope and a new direction for patients who have exhausted other treatment options.
Epigenetic Modulators: Targeting the Gene Expression Landscape
Epigenetic modulation represents another emerging frontier in the battle against mCRPC. By influencing gene expression rather than directly attacking the cancer, these therapies aim to alter the tumor microenvironment and potentially re-sensitize cancer cells to other treatment modalities.
Key considerations for epigenetic modulators include:
- Reversing Resistance: These drugs may help restore sensitivity to AR-directed therapies and chemotherapy.
- Combination Strategies: They are likely to be most effective when used in combination with other approved therapies.
- Underlying Mechanisms: Understanding the hidden complexities of gene regulation is on edge, and ongoing research is deciphering which patients might benefit the most.
Given the potential of epigenetic approaches, current research is geared toward establishing how these modulators can be integrated into existing treatment algorithms to maximize patient outcomes.
Biomarker-Driven Approaches: The Key to Personalized Medicine
In recent years, there has been a significant shift toward using biomarker-driven strategies to guide treatment decisions for mCRPC. Genomic profiling of tumors has uncovered a range of mutations and defective pathways that not only drive the disease but also offer clues regarding which treatments will be most effective.
Genomic Profiling and DNA Repair Defects
One of the most super important areas in mCRPC management is the identification of defects in DNA repair mechanisms—specifically in homologous recombination repair genes. Patients with these defects often respond well to PARP inhibitors and even show promise with other targeted therapies. The availability of genomic profiling allows clinicians to dig into these fine points for a more nuanced understanding of the disease.
Major benefits of genomic profiling include:
- Personalization: Tailoring treatments based on individual genetic mutations.
- Enhanced Efficacy: Maximizing treatment effectiveness by matching therapy to cancer’s genetic profile.
- Prognostic Value: Some genetic alterations can provide insight into likely outcomes and help set realistic expectations.
Tools like next-generation sequencing have not only improved our ability to detect subtle differences in patient tumors but also help in steering treatment decisions. The process is a classic example of how modern medicine is using science to figure a path through the maze of cancer complexity.
Mismatch Repair Deficiency and Immune Response
While DNA repair defects have taken center stage, another promising biomarker is mismatch repair deficiency. Tumors with these defects tend to be more visible to the immune system, offering a rationale for the use of immunotherapy in a subset of patients with mCRPC.
Key points regarding mismatch repair deficiency include:
- Predictive Value: Patients exhibiting these deficiencies may experience improved responses to immune checkpoint inhibitors.
- Therapeutic Implications: Incorporating immunotherapy in treatment plans may yield benefits for patients whose tumors are full of problems with DNA mismatch repair.
- Ongoing Research: Current studies are working to define the optimal treatment regimens that combine immunotherapy with other modalities for this patient subgroup.
Although this area is still emerging, the ability to integrate mismatch repair status into clinical practice is a step forward in providing truly individualized care for patients with advanced prostate cancer.
Liquid Biopsies and the Future of Real-Time Monitoring
The dynamic nature of mCRPC calls for monitoring techniques that are as responsive as the disease. Liquid biopsies, which analyze tumor-derived circulating DNA in the blood, offer a less invasive way to track changes over time. By routinely monitoring these biomarkers, clinicians can adapt treatment plans more flexibly and respond quickly to shifts in the cancer’s behavior.
Highlights of liquid biopsy technology include:
- Minimally Invasive: Blood tests provide an easier method compared to traditional tissue biopsies.
- Real-Time Insights: Offering a snapshot of the tumor’s genetic makeup as it evolves during treatment.
- Treatment Adjustment: Allowing doctors to make on-the-fly decisions when resistance mechanisms begin to emerge.
This approach provides reassurance for both patients and clinicians that rapid shifts in disease biology can be captured accurately, fostering timely adjustments in therapy.
The Importance of Sequencing in Multimodal Treatment Approaches
Even with a growing menu of therapeutic options, one of the most challenging and nerve-racking tasks is the proper sequencing of these treatments. Physicians must balance the timing of androgen receptor degraders, PARP inhibitors, radioligand therapy, and emerging immunotherapies in a way that maximizes benefit while minimizing potential side effects.
Sequencing Strategies: Combining Old and New Therapies
Current strategies for treatment sequencing are evolving rapidly. In practice, setting the right order for these agents involves a series of small distinctions and requires an individualized approach for each patient. Clinicians weigh several factors, including:
- Overall Tumor Burden: The extent and location of metastases may influence which therapy should be introduced first.
- Patient Health Status: Coexisting conditions and overall health shape the feasibility of certain treatments.
- Molecular Profile: Biomarkers and genetic profiles help dictate which agents may offer a superior benefit at any given time.
Some treatment sequence examples that are being actively discussed include:
| Scenario | Potential Sequence | Rationale |
|---|---|---|
| High AR Activity | AR-signaling inhibitors → AR degraders | Begin with established AR inhibitors and transition to degraders as resistance emerges |
| Defective DNA Repair | AR inhibitors → PARP inhibitors | Capitalize on DNA repair deficiencies to suppress resistance |
| Immune-Responsive Tumor | Immunotherapy ± T-cell engagers | Utilize biomarker insights for immunotherapy to boost immune-mediated clearance |
In each scenario, the small distinctions in a patient’s disease characteristics guide doctors in finding their path. The challenge remains to integrate all available data to develop a treatment plan that is both flexible and resilient.
Combination Therapy: Merging Multiple Approaches
Beyond sequencing individual therapies, combination treatment strategies offer another promising avenue. The hope is that by employing two or more treatments simultaneously, clinicians can tackle the troublesome parts of resistance from different angles. Combining therapies may help:
- Address alternative pathways simultaneously
- Reduce the likelihood that cancer cells will develop a single, dominant escape mechanism
- Potentially lower the doses required for each agent, reducing the risk of side effects
These combination strategies require careful coordination and close monitoring. They are still the subject of numerous clinical trials aimed at figuring a path to optimal treatment regimens that address both the overt and hidden complexities of the disease.
Challenges and Opportunities in Clinical Practice
One of the most overwhelming challenges in treating mCRPC is the need to manage a disease that adapts quickly and often in unpredictable ways. For clinicians, every patient encounter is a reminder that while significant progress has been made, the path ahead is still dotted with confusing bits and demanding decisions.
Understanding Resistance Mechanisms
The ability of prostate cancer cells to become resistant to even the most advanced therapies is full of problems that require ongoing investigation. These resistance mechanisms include:
- Mutations in the androgen receptor that allow continued signaling despite blockade
- Activation of alternate survival pathways that bypass AR dependence
- Epigenetic changes that alter gene expression and promote a resistant phenotype
The goal for the future is to develop methods for predicting which pathway a cancer might use to escape treatment. In doing so, clinicians can work through these issues in real time rather than after resistance has manifested clinically.
Patient Quality of Life and Side Effects Management
Not all challenges are purely biological. The nerve-racking aspects of treatment also include managing the side effects and overall quality of life for patients. Many therapies, while effective, come with a host of off-putting side effects that can make day-to-day living difficult for patients. To address these challenges, a comprehensive care model must be adopted that includes:
- Regular assessment: Frequent evaluations to adjust dosages or switch therapies as needed.
- Support services: Integrating nutritional, psychological, and rehabilitative support into the treatment plan.
- Communication: Encouraging open dialogue between patients and their care teams about the trade-offs between treatment efficacy and quality of life.
This holistic approach is essential—after all, optimizing treatment isn’t just about extending survival; it’s also about ensuring that patients maintain as much functionality and comfort as possible during their journey.
Bridging the Gap Between Research and Practice
A major challenge in the field is translating the promising results of early clinical trials into routine clinical practice. The journey from research to bedside is often loaded with issues such as:
- The need for standardized guidelines that consider the myriad small distinctions emerging from trials
- Training the next generation of clinicians to use advanced genomics and biomarker techniques
- Ensuring equity in access to these novel therapies across different populations
Addressing these challenges will require a collaborative effort among research institutions, regulatory agencies, and healthcare providers. The goal is to create a framework that not only pushes the boundaries of current science but also practically supports clinicians in making the best decision for their patients on a daily basis.
Future Directions: From Laboratory Bench to Patient Bedside
Looking ahead, the evolution of treatment for mCRPC will depend on continued investment in translational research, ongoing clinical trials, and the integration of cutting-edge technologies into routine practice. With the development of agents like AR degraders, ADCs, improved immunotherapies, and epigenetic modulators, the future appears hopeful, though not without its nerve-racking twists and turns.
Harnessing Data for Better Treatment Outcomes
One of the most promising aspects of current research is the use of big data and machine learning to predict treatment responses. By gathering data from thousands of patients, researchers are beginning to piece together patterns that could eventually help clinicians make more informed, personalized decisions. Key initiatives include:
- Predictive Algorithms: Using patient data to forecast which treatment sequences might work best for specific genetic profiles.
- Outcome Registries: Establishing large databases to track long-term outcomes of various treatment regimens.
- Collaborative Networks: Sharing data across institutions to refine and validate predictive models.
These data-driven approaches will be essential in finding the right mix and sequence of therapies that can successfully address both the obvious and hidden issues associated with mCRPC.
Improving Patient Engagement and Shared Decision-Making
As treatments become more personalized, the role of patient engagement in decision-making becomes increasingly important. The best outcomes in mCRPC management are achieved when clinicians and patients work closely together and weigh the advantages and disadvantages of each treatment option. This cooperative approach might involve:
- Decision Aids: Tools and resources that help patients understand the potential benefits and drawbacks of treatment options.
- Regular Consultations: Frequent discussions to update treatment plans in light of new research findings or changes in patient health.
- Holistic Care: Recognizing that treatment optimization extends beyond drug regimens to include physical, nutritional, and psychological support.
By fostering an environment where patients feel comfortable voicing their concerns and preferences, clinicians can ensure that treatment plans remain adaptive and responsive to the patient’s needs.
Integrating Novel Technologies into Routine Care
Finally, one of the most exciting frontiers is the potential integration of novel technologies—such as liquid biopsies, wearable health monitors, and telemedicine—into the management of mCRPC. These tools not only create opportunities for earlier detection of resistance but also allow for real-time adjustments in therapy. The technological advances on the horizon promise to help us:
- Monitor changes in tumor biology with minimal invasiveness
- Enhance communication between patients and healthcare providers
- Streamline the process of data collection, ultimately informing better treatment decisions
The integration of these technologies represents a shift toward a more proactive, patient-centered model of care that could revolutionize the overall management of mCRPC.
Conclusion: Charting a Course Through a Dynamic Landscape
The treatment of metastatic castration-resistant prostate cancer is a field marked by its tricky parts, unexpected turns, and endless opportunities for improvement. While the current arsenal of FDA-approved therapies—including AR-signaling inhibitors, PARP inhibitors, radioligand therapy, and immunotherapy—has provided a solid foundation, many aspects of treatment remain complicated and on edge. Clinicians are continuously challenged by issues such as resistance mechanisms, optimal sequencing, and ensuring quality of life for their patients.
Emerging strategies such as androgen receptor degraders, antibody-drug conjugates, T-cell engaging immunotherapies, and epigenetic modulators promise to address some of these challenges. At the same time, biomarkers derived from genomic profiling, mismatch repair deficiency analyses, and liquid biopsies are beginning to transform how personalized medicine is delivered. These advances help us figure a path that is both innovative and responsive to the unique needs of each patient.
The future of mCRPC management hinges on the collaborative integration of cutting-edge research, real-time data analytics, and patient-centered care practices. Although the road is full of problems and off-putting uncertainties, the potential to extend both the length and quality of life for patients is a reward that continues to motivate the global research and clinical communities.
Ultimately, optimizing treatment for mCRPC is not solely a matter of selecting the right drug—it is about tailoring a multimodal, carefully sequenced approach that anticipates resistance and adapts to the dynamic nature of the disease. Every patient’s journey is unique, and by embracing the latest advances and working through the subtle details that define each case, clinicians can guide their patients through the complicated pieces of mCRPC treatment toward improved outcomes.
In the realm of metastatic castration-resistant prostate cancer, there is no single perfect formula. Instead, success lies in the willingness to explore new therapies, integrate detailed biomarker insights, and remain flexible as new evidence emerges. As we look forward to a future where real-time monitoring, innovative technologies, and robust data analytics become the norm, one thing remains clear: while the challenges are many and every twist and turn is nerve-racking, the continuous quest for better, more personalized treatment provides hope and direction for both patients and clinicians alike.
By maintaining a neutral stance, recognizing the complex interplay of factors involved, and staying current with the latest research trends, the medical community can work through these tangled issues. The journey is long, and the details are fine and subtle, but every step we take in refining treatment strategies brings us closer to a future where personalized medicine for mCRPC is not just an aspiration but a standard of care.
In conclusion, optimizing treatment for metastatic castration-resistant prostate cancer entails managing a host of challenging factors—from well-established therapies to emerging strategies that are still in the clinical trial phase. By carefully evaluating the available options, aligning them with patient-specific biomarkers, and continuously adapting to new insights, clinicians can better steer through the nerve-racking landscape of advanced prostate cancer care. The path is difficult, the issues are complicated, but the ongoing innovation in treatment strategies is poised to bring hope and improved quality of life to patients facing this daunting diagnosis.
Originally Post From https://pubmed.ncbi.nlm.nih.gov/40782341/
Read more about this topic at
A New Precision Treatment for Prostate Cancer Preserves …
Precision Medicine Meets Prostate Cancer